Three Research Tools for Neurodegenerative Diseases

Three active, pathology-inducing proteins for drug discovery and model development in neurodegenerative diseases
Neurodegeneration disease is an umbrella term for a broad group of progressive neurological disorders that are characterized by neurons becoming damaged or dying. As neurons do not readily reproduce, these diseases of the nervous system are debilitating and incurable.
Neurodegenerative diseases can impair movement (ataxia) or mental functioning (dementia). The risk for acquiring a neurodegenerative disease increases with age due to heightened levels of oxidative stress and DNA mutations. Neurodegenerative disease includes Alzheimer’s disease (AD), Parkinson’s disease (PD), Amyotrophic lateral sclerosis (ALS), Friedreich’s ataxia, Huntington’s disease (HD), Lewy body dementia, etc.
AD and PD as the most common neurodegenerative disorders are both characterized by the presence of plaques in the brain – these consist of protein aggregates which include the key proteins: alpha synuclein, Amyloid beta and Tau. By using active, pathology-inducing aggregates as reagents, our scientists can develop reliable disease models and accelerate drug discovery.
1. Alpha Synuclein
Alpha Synuclein is a highly soluble, small protein without intrinsic structure, abundantly present in the brain, primarily in presynaptic terminals of neurons. It is involved in synaptic vesicle release for signaling, including dopamine, which is important for movement. Alpha synuclein has been shown to aggregate into various oligomeric and fibrillary forms and soluble, misfolded alpha synuclein aggregates are neurotoxic and can spread disease in a prion-like fashion. “Synucleinopathies” are a group of neurodegenerative diseases that includes Parkinson’s disease (PD), dementia with Lewy bodies (DLB), diffuse Lewy body disease (DLBD), and multiple system atrophy (MSA), which are all caused by misfolded alpha synuclein. Indeed, alpha synuclein aggregation is a hallmark of Parkinson’s disease and different forms of the protein can be used to develop disease models that reproduce the pathological features of this disorder.
Biohippo carries a wide variety of monomeric, oligomeric and fibrillar alpha synuclein constructs for neurodegenerative disease research. These preparations have different characteristics and properties.
1.1 Alpha Synuclein Pre-formed Fibrils (PFFs)Alpha synuclein pre-formed fibrils (PFFs) seed the formation of new fibrils from active alpha synuclein monomers and can be used to induce endogenous alpha synuclein phosphorylation and subsequent Lewy body inclusion formation in neuronal cell culture or for in vitro oligomerization studies. Alpha Synuclein Pre-formed Fibrils (PFFs) (BHP11900142) were shown to be taken up by SH-SY5Y cells and transmitted to neuronal iPSCs within 14 days.
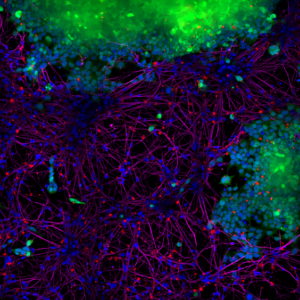
Picture 1: Blue: Hoechst/DNA | Green: SHSY5Y-GFP | Red: StressMarq’s α-syn PFFs-555 | Purple: Tubulin
1.2 Alpha Synuclein Oligomers
Alpha synuclein oligomers are increasingly thought to be the toxic species in synucleinopathies. Kinetically stable alpha synuclein oligomers (BHP11901215) are toxic to dopaminergic neurons and induce phosphorylation of alpha synuclein Ser129 (a pathology associated with Parkinson’s disease) and are generated without the addition of any inducers or inhibitors. We also carry dopamine-stabilized alpha synuclein oligomers (BHP11901184) and EGCG-stabilized alpha synuclein oligomers (BHP11901187). Dopamine and EGCG can be used to stabilize alpha synuclein in its oligomeric forms, preventing further aggregation into fibrils.
|
Alpha Synuclein Oligomers |
SKU |
1.3 Alpha Synuclein Monomers
Alpha synuclein monomers are capable of aggregation and do not show neurodegenerative activity.
2. Amyloid Beta
In the brain, amyloid beta peptide (Aβ) is generated by protease cleavage of amyloid precursor protein (APP), which aggregates into oligomers, protofibrils, fibrils and ultimately plaques in neurodegenerative diseases. The accumulation of Aβ plaques in the brain is considered a hallmark of Alzheimer’s disease (AD), and most of the drugs tested for AD in the past 20 years have targeted amyloid beta accumulation. Soluble Aβ oligomers isolated from the brains of AD patients or those generated in vitro potently impaired synapse structure and function. Aβ oligomers generated in vitro were toxic to PC12 cells and SH-SY5Y cells. Aβ was demonstrated to interact with tauopathies to affect neurodegeneration in AD patients and accumulations of Aβ were shown to be associated with lower survival rates in Parkinson’s disease patients with dementia.
| Amyloid Beta | SKU |
| Human Amyloid Beta 1-42 Pre-formed Fibrils (PFFs) | BHP11901217 |
| Human Amyloid Beta 1-42 Oligomers | BHP11901218 |
| Human Amyloid Beta 1-42 Peptide (HFIP, monomeric) | BHP11901216 |
3. Tau Pre-formed Fibrils (PFFs), Filaments, & Monomers
Active tau proteins can be used to study tau aggregation, a hallmark of neurodegenerative diseases including Alzheimer’s. The process of tau aggregation can be seeded by active tau PFFs, which recruit monomers to form larger tau fibrils. This has been demonstrated in thioflavin T assays where an increase in fluorescence – indicative of tau fibrillization – is seen when active tau PFFs are combined with active tau monomers. Certain tau PFF have been injected into P301L mice, where they seed tau aggregation and induce tau pathology in the hippocampus (Picture 2).
3.1 Tau Pre-formed Fibrils (PFFs)
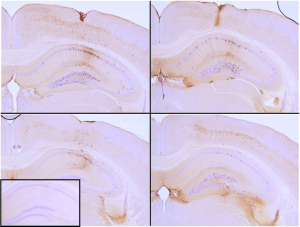
Picture 2: Immunohistochemistry analysis of P301L mouse hippocampus injected with K18 P301L tau PFFs (BHP11900160) shows seeding of tau pathology at injection site nine weeks post-injection. AT8 (pSer202/pThr205) tau antibody shows tangle-like inclusions. Inset: negative control. Experiments performed at reMYND N.V.
3.2 Tau Monomers
|
Tau Monomers |
SKU |
|
Human Tau-441 (2N4R) P301S Mutant Monomers (Sf9/Baculovirus) |
|
|
Human Tau Truncated Fragment (AA297-391) (dGAE C322A) Monomers |
|
All the above products are available at Biohippo. Our supplier, StressMarq, is the only commercial company with a wide and validated PPF/oligomer portfolio. If you have any questions, please feel free to contact us at [email protected]. For more information, please visit our website: https://www.ebiohippo.com/en/store/stressmarq/.
 Loading ....
Loading ....
