ELISA Myth-Busting Series
__1501774255.jpg)
Busting the Myths Around ELISA Kits
The Enzyme-Linked Immunosorbent Assay (ELISA) is one of the most commonly used laboratory techniques, yet misinformation about its use persists. With decades of proven reliability, ELISA is still a fundamental tool in research and diagnostics. In this overview, we'll address misconceptions that often hinder successful experimentation and reveal best practices for getting the most out of your ELISA kits.
The Enzyme-Linked Immunosorbent Assay (ELISA) is a powerful tool for detecting and quantifying proteins, antigens, and other molecules. However, despite its widespread use, there are still several myths and misconceptions surrounding ELISA kits that can lead to confusion or even hinder effective experimental design. In this series, we aim to debunk these myths and provide accurate, reliable information to help researchers make informed decisions.
Myth #1: ELISA Kits Are Always Plug-and-Play

Summary: ELISA kits may seem straightforward, but they require precise handling to achieve reliable outcomes.
Reality: While ELISA kits are often marketed as user-friendly and ready-to-use, successful results still require careful attention to detail. Factors like sample preparation, consistent incubation temperature, incubation times, and reagent handling are crucial for reliable results. Following each step meticulously and adapting protocols to suit your specific application is essential for achieving reliable results.
Tip: Always read the kit instructions thoroughly before starting, and plan the experiment step-by-step to ensure nothing is overlooked.
Myth #2: All ELISA Kits Have the Same Sensitivity and Specificity
.jpg) Summary: Not all ELISA kits perform equally; variations in quality impact their sensitivity and specificity.
Summary: Not all ELISA kits perform equally; variations in quality impact their sensitivity and specificity..jpg)
Reality: Not all ELISA kits are created equal. The sensitivity and specificity of an ELISA kit depend heavily on the quality of the antibodies used, the robustness of the assay design, and how well the assay is optimized. Researchers should carefully evaluate validation data and match the kit’s sensitivity to their specific research needs.
.jpg)
Tip: Always check the kit’s datasheet for sensitivity and specificity details, and consider running pilot tests to confirm its performance for your specific application.
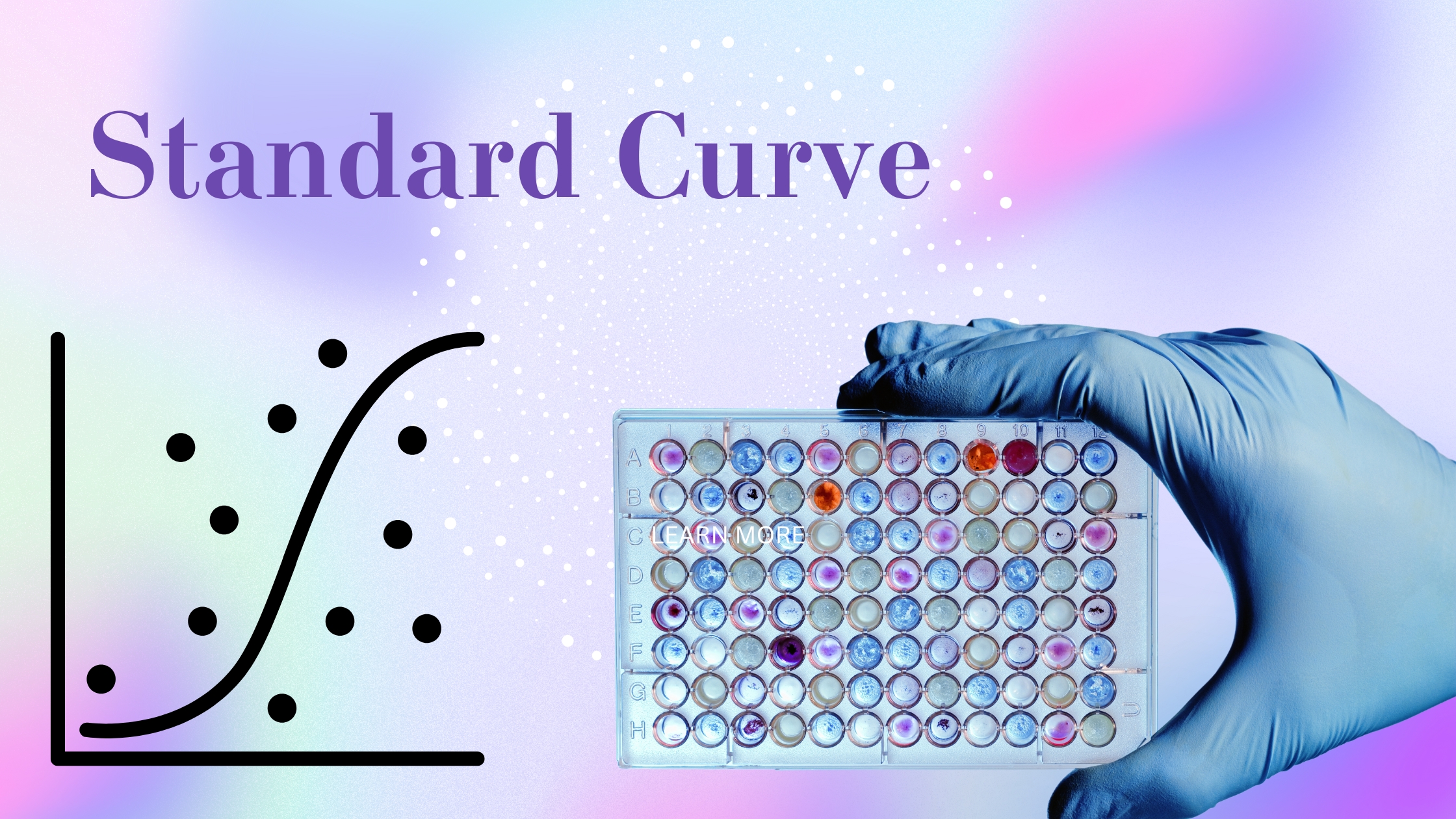
Myth #3: A Standard Curve Is Always Linear
Summary: ELISA standard curves can take different shapes, and assuming linearity can lead to incorrect data interpretation.
Reality: It’s a common misconception that the standard curve in an ELISA must be linear. In fact, many ELISA assays produce a sigmoidal standard curve. Understanding the appropriate curve-fitting model is critical to accurately quantify your results. Always ensure you use the proper model for your specific assay.
Tip: Use software to fit your data to the appropriate model, whether it’s linear, sigmoidal, or another fit, to ensure the most accurate quantification.
Myth #4: Washing Steps Are Not That Important
.jpg) Summary: Proper washing is essential for reducing background noise and achieving accurate results.
Summary: Proper washing is essential for reducing background noise and achieving accurate results..jpg)
Reality: The washing steps are actually one of the most critical parts of an ELISA protocol. Inadequate washing can lead to high background or false positives due to unbound reagents that remain in the well. Consistency in washing procedures, such as using an automated plate washer, can significantly improve assay performance.
.jpg)
Tip: Make sure to use a consistent washing protocol, including the recommended number of washes and adequate buffer volume, to minimize background signals.
Myth #5: ELISA Can Be Used for Any Sample Type Without Modification
Summary: Different sample types often require specific modifications to the ELISA protocol for accurate results.
Reality: Different sample types (e.g., serum, plasma, tissue lysate) may require modifications to the standard ELISA protocol. Factors like sample matrix effects and interfering substances can significantly affect the results. It's essential to perform optimization for your specific sample type to ensure accuracy.
Tip: Perform a sample matrix analysis and consider diluting or modifying the sample preparation to minimize interference and improve assay results.
Myth #6: ELISA Is Outdated Compared to Newer Technologies
.jpg) Summary: Despite newer technologies, ELISA remains a gold standard for its simplicity and reliability.
Summary: Despite newer technologies, ELISA remains a gold standard for its simplicity and reliability..jpg)
Reality: Although newer technologies like multiplex assays and next-generation sequencing are emerging, ELISA remains a gold standard due to its simplicity, robustness, and cost-effectiveness. ELISA is still widely used in both research and clinical diagnostics, and its reliability continues to make it a trusted method for quantifying analytes.
.jpg)
Tip: Consider using ELISA when you need a proven, cost-effective, and reliable method for quantification, especially when high-throughput or specialized equipment is not available.
Myth #7: Blocking Steps Are Optional
Summary: Blocking is a crucial step to ensure specificity in ELISA.
Reality: Blocking is a crucial step in the ELISA protocol to prevent non-specific binding, which could lead to false results. Skipping or inadequately performing the blocking step can compromise the reliability of your data. It is essential to select an appropriate blocking buffer and ensure sufficient blocking time.
Tip: Always choose a blocking buffer that is compatible with your assay and allow adequate blocking time to reduce background noise.
Myth #8: More Antibody Always Means Better Signal
.jpg) Summary:Excessive antibody concentration can lead to non-specific binding.
Summary:Excessive antibody concentration can lead to non-specific binding..jpg)
Reality: Using higher concentrations of antibodies does not necessarily lead to better signals and can sometimes cause issues such as high background noise or non-specific binding. It’s important to use optimized antibody concentrations as per the kit’s protocol to achieve the best signal-to-noise ratio.
.jpg)
Tip: Optimize antibody concentrations by following the kit recommendations or running a titration experiment to find the optimal balance between signal strength and specificity.
Myth #9: ELISA Is Only Useful for Protein Detection
Summary: ELISA can detect a variety of molecules beyond proteins.
Reality: While protein detection is one of the primary applications of ELISA, it can also be used for detecting hormones, peptides, antibodies, and even small molecules. The versatility of ELISA makes it applicable to a wide range of research areas beyond protein quantification.
Tip: Consider ELISA for applications such as hormone assays or antibody quantification, expanding its use beyond just protein detection.
Myth #10: Results Are Always Accurate Without Replicates
.jpg) Summary:Running replicates is essential for accuracy and reliability.
Summary:Running replicates is essential for accuracy and reliability..jpg)
Reality: Running replicates is crucial for obtaining reliable ELISA results. Without replicates, it’s impossible to identify outliers or assess the precision of your assay. Performing experiments in duplicate or triplicate helps ensure the reliability and reproducibility of the data.
.jpg)
Tip: Always run your samples in duplicate or triplicate to detect any anomalies and confirm the precision of your results.
Myth #11: Higher Temperature Always Improves Reaction Speed
Summary: Elevated temperature can harm assay performance if not controlled.
Reality: While temperature can influence reaction kinetics, increasing temperature indiscriminately can also lead to protein denaturation or reduced specificity in ELISA assays. It is crucial to follow the temperature recommendations in the protocol to avoid compromising the assay quality.
Tip: Stick to the recommended temperature for incubation to maintain enzyme activity and avoid compromising the assay results.
Myth #12: ELISA Plates Are Reusable
.jpg) Summary: Reusing plates compromises assay accuracy.
Summary: Reusing plates compromises assay accuracy..jpg)
Reality: ELISA plates are designed for single use, and attempting to reuse them can lead to cross-contamination and unreliable results. The coating and blocking steps are not easily reversible, making reuse impractical and potentially inaccurate.
.jpg)
Tip: Always use fresh plates for each assay to avoid cross-contamination and ensure consistent results.
Myth #13: All Blocking Buffers Are Interchangeable

Summary: Not all blocking buffers yield the same results.
Reality: Not all blocking buffers work equally well for every ELISA. Depending on the nature of the analyte and the antibodies used, some blocking buffers can lead to increased background or non-specific binding. It is essential to test and optimize the blocking buffer for each specific assay.
Tip: Test different blocking buffers during assay development to determine which yields the lowest background for your specific application.
Myth #14: Substrate Incubation Time Doesn't Affect Sensitivity
.jpg) Summary: Incubation time is crucial for optimal sensitivity.
Summary: Incubation time is crucial for optimal sensitivity..jpg)
Reality: The incubation time for substrates can significantly affect the sensitivity of your ELISA. Shorter incubation times might not allow for full color development, while overly long times can lead to higher background. Careful optimization of substrate incubation is key to obtaining reliable data
.jpg)
Tip: Optimize substrate incubation times by running a time-course experiment to determine the ideal duration for maximum signal without increased background.
Myth #15: Signal Amplification Always Improves Detection

Summary: Amplification can also increase background noise.
Reality: Signal amplification can increase sensitivity, but it also has the potential to amplify noise, leading to false positives or high background. It’s important to balance signal amplification with specificity to maintain reliable results.
Tip: Use signal amplification only when necessary and always include proper controls to differentiate between true signals and amplified noise.
Myth #16: Any Plate Reader Can Be Used for ELISA
.jpg) Summary: Plate readers vary in their suitability for ELISA.
Summary: Plate readers vary in their suitability for ELISA..jpg)
Reality: Not all plate readers are optimized for ELISA detection. Differences in sensitivity, wavelength accuracy, and calibration can affect data quality. Using a plate reader specifically validated for ELISA is essential for reproducible results.
.jpg)
Tip: Ensure your plate reader is calibrated for ELISA and has the appropriate filters for the detection wavelengths recommended in your assay protocol.
Myth #17: Using Tap Water for Washing Is Acceptable
Summary: Tap water impurities can affect results.
Reality: Tap water contains impurities and minerals that can interfere with ELISA reactions, leading to inconsistent results. Always use deionized or distilled water for washing steps to ensure assay reliability.
Tip: Use deionized or distilled water with proper buffer solutions for all washing steps to maintain assay consistency and accuracy.
Myth #18: Antibody Cross-Reactivity Is Not a Major Concern
.jpg) Summary: Cross-reactivity can compromise ELISA accuracy.
Summary: Cross-reactivity can compromise ELISA accuracy..jpg)
Reality: Cross-reactivity can significantly affect ELISA accuracy by leading to false positives. It's essential to verify the specificity of antibodies, especially in complex sample matrices, to avoid misleading results
.jpg)
Tip: Use highly specific antibodies and consider performing pre-absorption steps to minimize cross-reactivity
Myth #19: Color Intensity Always Indicates Analyte Concentration
Summary: Color intensity can be influenced by various factors.
Reality: While color intensity generally correlates with analyte concentration, factors such as substrate stability, incubation times, and enzyme activity can influence signal intensity. Proper standard curve analysis is essential to accurately interpret results.
Tip: Always use a standard curve to quantify analyte concentrations accurately, rather than relying solely on color intensity.
Conclusion
Setting the Record Straight on ELISA Kits
We hope this series will not only debunk misconceptions but also enhance your confidence in using ELISA kits for your research.
Take the Next Step
If you're ready to get the most out of your ELISA experiments, browse our upcoming detailed blog posts for deeper insights into each myth. Additionally, explore our complete range of ELISA kits to find the right fit for your research needs: Browse Our ELISA Kits. Sign up for our newsletter to get notified when new myth-busting content is available, and don't hesitate to reach out if you have specific questions or need support with your assays.
Ready to Dive Deeper?
Explore our detailed posts that dissect each myth individually, providing more comprehensive explanations, examples, and practical tips. By debunking these common myths, we hope to provide clarity and empower researchers to use ELISA kits more effectively. Understanding the realities behind these misconceptions can lead to more accurate experiments, fewer frustrations, and better research outcomes. Stay tuned forsthe upcoming blogs that will explore each myth in detail! For further in-depth discussions, explore the links to each myth in our series.
 Loading ....
Loading ....
