Integrated AAV Offerings: From Cutting-Edge Products to Advanced Custom Services
Overcoming AAV Technology Challenges with Expertly Tailored Vector Design and Production
AAV Serotypes: An Overview
Adeno-associated virus (AAV) vectors are widely used in gene therapy due to their efficient transduction, long-term gene expression, low immunogenicity, and favorable safety profile. Each AAV serotype has distinct capsid proteins that determine its tropism—its preference for infecting specific cell types or tissues. To date, at least 13 natural AAV serotypes and over 100 variants have been identified, many of which have received regulatory approval for therapeutic use. These serotypes exhibit diverse tissue tropism and utilize a range of receptors and co-receptors for cellular entry.
Approved AAV-based Gene Therapies
| Drug | Disease | AAV Serotype | Target | Approval |
|---|---|---|---|---|
| Glybera | LPLD | AAV1 | Muscle | EMA, 2012 |
| Luxturna | RPE65-LCA | AAV2 | Retina | FDA, 2017 |
| Zolgensma | SMA | AAV9 | Brain | FDA, 2019 |
| Upstaza | AADC | AAV2 | Brain | EMA, 2022 |
| Roctavian | Hemophilia A | AAV5 | Liver | EMA, 2022 |
| EtranaDez | Hemophilia B | AAV5 | Liver | FDA, 2022 |
| ELEVIDYS | DMD | AAVrh74 | Muscle | FDA, 2023 |
AAV Receptors and Co-receptors
AAV serotypes utilize different primary receptors and co-receptors to mediate cell entry, which influences their tropism and transduction efficiency:
| Serotype | Primary Receptor | Co-receptor |
|---|---|---|
| AAV1 | α2-3 N-linked SIA and AAVR | Unknown |
| AAV2 | HSPG and AAVR | FGFR1, αVβ5 integrin, α5β1 integrin, HGFR, LR, CD9 |
| AAV3/AAV3B | HSPG and AAVR | FGFR1, HGFR, LR |
| AAV4 | α2-3 O-linked SIA | Unknown |
| AAV5 | α2-3 N-linked SIA and AAVR | PDGFRα and PDGFRβ |
| AAV6 | HSPG, α2-3 and α2-6 N-linked SIA, AAVR | EGFR |
| AAV7 | Unknown | Unknown |
| AAV8 | LR and AAVR | Unknown |
| AAV9 | Terminal N-linked galactose, AAVR | LR, putative integrin |
| AAV11 | Unknown | Unknown |
| AAV12 | Putative mannose and mannosamine | Unknown |
| AAV13 | Unknown | HSPG |
| AAVrh.10 | LacNAc and AAVR | Unknown |
Pre-made AAV Serotype Testing Kits
AAV Serotype Testing Kits provide a quicker method to identify a specific subset of AAV serotypes by measuring the expression levels of fluorescent proteins (EGFP, mCherry, TdTomato) or luciferases (Gluc, Rluc, Fluc, Cluc). This approach is particularly useful when researchers have a clear understanding of the target cell lines or tissues and seek a streamlined way to narrow down their choices to a few optimal AAV serotypes.
In this category, 16 most used AAV serotypes (AAV1, AAV2, AAV3B, AAV5, AAV6, AAV7, AAV8, AAV9, AAV11, AAV12, AAV13, AAV-DJ, AAVrh.10, AAVrh.74, and AAV2-Retro, AAV4-Y729F) are included in each kit. The vectors carry fluorescent protein (EGFP, mCherry, TdTomato) or reporter luciferase (Fluc, Rluc, Cluc, Gluc), which is driven by a promoter (CAG, CMV, EF1a or hSyn).
| SKU | Name | Feature System | Scale | Price |
|---|---|---|---|---|
| PK0001 | AAV Serotype Testing Kit-CAG-EGFP | CAG-EGFP | 50uL x 1E+13 GC/mL | $1,199 |
| PK0002 | AAV Serotype Testing Kit-CAG-mCherry | CAG-mCherry | 50uL x 1E+13 GC/mL | $1,199 |
| PK0003 | AAV Serotype Testing Kit-CMV-mCherry | CMV-mCherry | 50uL x 1E+13 GC/mL | $1,199 |
| PK0004 | AAV Serotype Testing Kit-CAG-TdTomato | CAG-TdTomato | 50uL x 1E+13 GC/mL | $1,199 |
| PK0005 | AAV Serotype Testing Kit-CMV-Fluc | CMV-Fluc | 50uL x 1E+13 GC/mL | $1,199 |
| PK0006 | AAV Serotype Testing Kit-EF1a-EGFP | EF1a-EGFP | 50uL x 1E+13 GC/mL | $1,199 |
| PK0007 | AAV Serotype Testing Kit-EF1a-mCherry | EF1a-mCherry | 50uL x 1E+13 GC/mL | $1,199 |
| PK0009 | AAV Serotype Testing Kit-hSyn-mCherry | hSyn-mCherry | 50uL x 1E+13 GC/mL | $1,199 |
Notes:
1. All AAV viruses have full particle ratios >80%, measured by mass photometry.
2. We also provide vustomized AAV serotype testing kits packaging service.
AAV Tropism Analysis with Barcode-seq Technology
Barcode-seq Technology for AAV Evaluation
With the diversity of natural AAV serotypes and the continuous engineering of novel variants, the quest to design tailor-made capsids that can effectively target specific cell types and tissues is crucial for the success of gene therapies. However, the traditional methods of evaluating AAV vectors—such as qPCR, blotting techniques, RT-qPCR, fluorescent reporter assays, and immunohistochemistry—often require significant effort and resources, especially when dealing with a large number of AAV variants. These methods are also prone to inconsistencies across experiments and can limit the exploration of superior AAV candidates.
To overcome these challenges, Barcode-seq technology has been developed as a high-throughput and cost-efficient method for simultaneously comparing multiple AAV variants. This innovative approach allows for the parallel evaluation of numerous AAV capsid or promoter variants, providing a streamlined process to identify the most effective AAVs for specific therapeutic applications.
Barcode-seq (Barcode Sequencing) leverages the capabilities of deep sequencing platforms, such as Illumina’s amplicon-seq, to evaluate AAV vectors. In this method, AAV capsid or promoter variants are encoded with unique DNA sequences called barcodes. These barcodes act as unique identifiers that allow researchers to track and quantify the transduction efficiency and expression levels of each AAV variant in cells or tissues. To minimize any potential influence that barcodes may have on transgene expression, each AAV variant is typically paired with multiple barcodes.
How Barcode-seq Technology Works
AAVnerGene's ATHENA I Known Capsid Library utilizes a single capsid variant equipped with three distinct barcodes. By analyzing the frequency of these barcodes in the sequencing results, researchers can deduce the transduction efficiency and expression level of each corresponding AAV variant.
Case 1: AAV Serotypes and Their Tropism in C57B6 Mouse
AAV Capsid Library(I) was systematically injection into C57B6 mouse. All tissues were collected at 2 weeks post injection. All data shows the enrichment folds of barcodes (BCs) in tissues relative to the input viruses, obtained by NGS analysis.

Conclusion:
RNA Level: The AAV serotype with the best overall performance in different tissues is AAV9, followed by AAVrh.10.
DNA Level: The two AAV serotypes with the best overall performance are AAV-DJ and AAVrh.74.
| Tissue | Top Performing Serotypes (DNA Level) | Top Performing Serotypes (RNA Level) |
|---|---|---|
| Brain | AAVrh.74, AAV9, AAVrh.10, AAV11 | AAV9, AAVrh.10 |
| Heart | AAVrh.74, AAV11, AAV-DJ, AAVrh.10, AAV8 | AAV-DJ, AAV9, AAVrh.10 |
| Kidney | AAV-DJ, AAV8, AAV3B, AAVrh.74 | AAV9, AAV8, AAVrh.10, AAV7 |
| Liver | AAV-DJ, AAVrh.74, AAV8 | AAV8, AAVrh.10, AAV-DJ |
| Lung | AAV11, AAV5 | AAV9, AAV11, AAVrh.10 |
Case 2: AAV Tropism in Different Brain Regions(B6C3 mouse)
Ranking of RNA expression of 15 commonly used AAV serotypes in different regions of B6C3 brain after systematically injection of AAV Capsid Library(I).
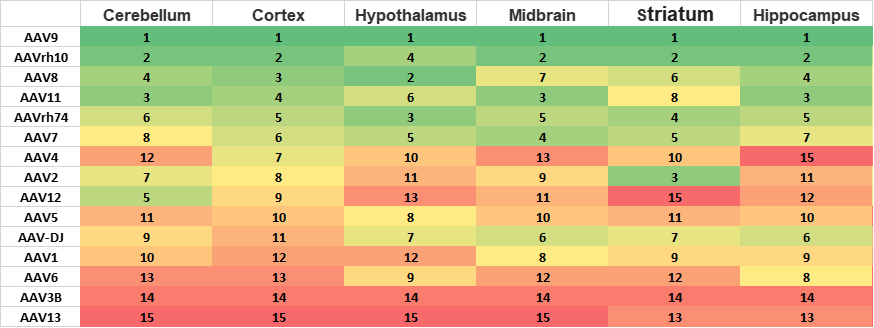
Conclusion:
The AAV9 is the best AAV serotypes with ability to crossing blood brain barrier (BBB) in B6C3 mice. It ranks in 1 for cerebellum, cortex hypothalamus, midbrain, striatum and hippocampus. AAV2 may have relative specific expression in Striatum.
Case 3: Performance of Crossing BBB Capsids in B6C3 Mice
AAV Capsid Library(I) was systematically injection into B6C3 mouse. All tissues were collected at 2 weeks post injection. All data shows RNA expression folds compared with AAV9.

Conclusion:
Among the tested AAV capsids, AAV9P31 demonstrated the highest efficiency in most brain tissues (cerebellum, cortex hypothalamus, midbrain, striatum and hippocampus), followed by AAV-F, AAV-MDV1A, AAV-PHP.C2, AAV-PHP.eB, AAV-PHP.B, and AAV-PHP.B3.
scRNA-AAVseq for AAV Tropism Analysis
Analyzing AAV tropism in different tissues is challenging due to the complexity of cell types and the diversity of hundreds of AAV serotypes and variants. To address these challenges, Biohippo, in collaboration with AAVnerGene, has developed a novel pipeline called scRNA-AAVseq (single-cell RNA-AAV barcode sequencing). This advanced technique allows for in vivo characterization of barcoded AAV pools at an unprecedented single-cell resolution, enabling a deep and detailed understanding of AAV tropism across various tissues and cell types.
Overview of the scRNA-AAVseq Workflow
Injection of Barcoded AAV Variants:
Multiple barcoded AAVs are pooled and injected into the target organism, such as mice, via retro-orbital injection. This enables the distribution of different AAV variants across multiple tissues and cell types.
Tissue Dissociation and Single-Cell Isolation:
After sufficient expression time, tissues (e.g., cortex) are harvested and dissociated into single-cell suspensions. This step involves tissue dissociation, preparation of single-cell suspensions, and droplet-based sorting to isolate single cells.
Library Preparation and Sequencing:
Single-cell libraries are prepared using droplet-based sequencing platforms such as Illumina NGS. Both cell transcriptomes and viral variant transcripts are processed for sequencing.
The transcriptome aliquots undergo the standard library construction protocol, further amplified using AAV barcode-specific primers to enrich AAV capsids. These amplified viral transcripts are then sequenced separately to generate NGS libraries.
Data Processing and Analysis:
The single-cell transcriptome analysis is performed using CeleScope 2.0 and annotated with in-house software. The sequencing reads from amplified viral transcripts are used to quantify the abundance of each viral barcode associated with each cell barcode and unique molecular identifier (UMI).
The most abundant viral barcode for each cell barcode and UMI is assumed to be the correct viral barcode, which is then used to construct a variant lookup table for further analysis.
AAV Capsid Library Evolution
We can do AAV capsid evolution both in vitro (primary cells and cell lines) and in vivo (mouse, rat, pig and NHP). Please discuss with our technical support for your screening needs.
Contact UsAAV Services
AAV Vector Design & Cloning
Expert consultation, design, gene synthesis, and cloning of custom AAV vectors tailored to your research needs.
Learn moreAAV Packaging Service
Reliable, affordable, and fully customizable AAV packaging service with diverse serotype options and high titer.
Learn moreAAV QC & Characterization
Comprehensive quality control assays including qPCR, SDS-PAGE, mass photometry, and next-generation sequencing.
Learn moreOther Viruses at Biohippo
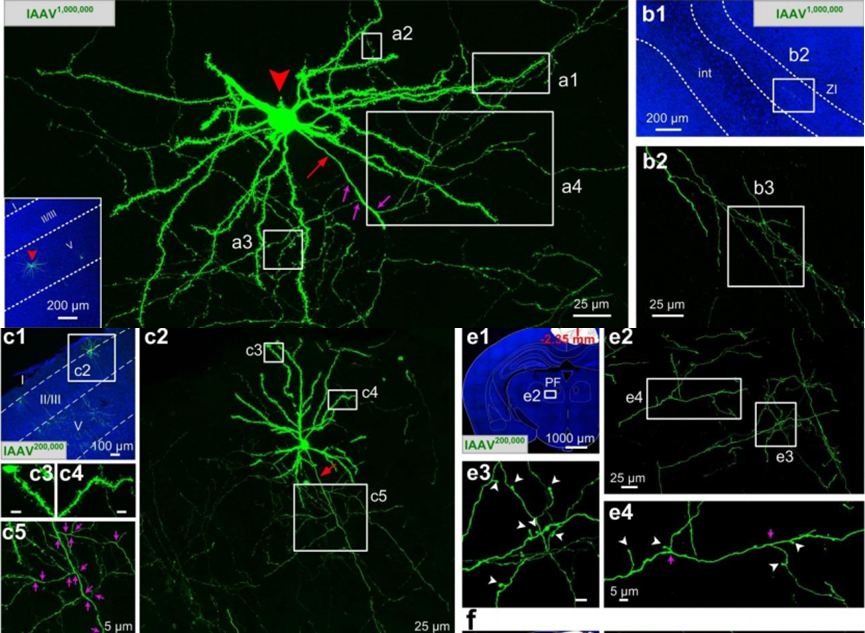
Neural Circuit Tracing
Biohippo offers a variety of viral vectors, including RV, HSV, PRV, and VSV, to replace conventional tracers with the highly efficient, more specific, and less invasive viral method.
Learn More
Lentivirus
Biohippo offers TF reporter lentivirus, immunotherapy lentivirus, validated shRNA, cDNA, and miRNA lentiviruses to meet diverse research needs. We also offer customized service at affodable price.
Learn More Loading ....
Loading ....
