Integrated AAV Offerings: From Cutting-Edge Products to Advanced Custom Services
Overcoming AAV Technology Challenges with Expertly Tailored Vector Design and Production
AAV Capsids for Non-Human Primates (NHPs)
AAV Cross-Species Issues
Animal models are fundamental for screening and optimizing AAV capsids targeting specific tissues. However, a critical insight from recent studies is that AAVs often display significant performance differences across species or even within different strains of the same species. For example, AAV-PHP.B, known for its ability to cross the blood-brain barrier (BBB), shows this property only in certain mouse strains and not in non-human primates (NHPs)【Hordeaux et al., 2018; Matsuzaki et al., 2019】. To improve the chances of translating AAV variants from animal models to human applications, it is essential to conduct variant screening across multiple animal models and human-based systems, focusing on those with translatable performance【Tabebordba et al., 2021; Gonzalez et al., 2022】.
Directed Evolution of AAV Variants for CNS Targeting
Directed evolution of a random peptide insertion library based on the AAV9 capsid's VR-VIII region led to the creation of the AAV-PHP family, including AAV-PHP.B, which significantly enhances BBB crossing in mice. Subsequent efforts to re-diversify this family produced AAV-PHP.eB, which maintains the peptide insertion of AAV-PHP.B but adds flanking substitutions. This modified variant demonstrated more efficient CNS transduction in mice, achieving a 55–76% transduction rate for neurons, a more than 2.5-fold increase over AAV-PHP.B.
Further engineering of AAV-PHP.eB by modifying the VR-IV region resulted in liver-detargeted variants like AAV.CAP-B10 and AAV.CAP-B22:
AAV.CAP-B10: This variant retains CNS targeting in mice but shows decreased transduction of peripheral organs and a significant reduction (50-fold) in liver tropism following IV delivery compared to AAV-PHP.eB. It exhibits robust CNS transgene expression in marmosets, achieving a 4-fold increase over AAV9, with 17-fold lower liver expression.
AAV.CAP-B22: Exhibits 12-fold higher CNS transduction in marmosets than AAV9, with increased astrocyte transduction. However, it does not translate effectively in newborn rhesus macaques, highlighting the challenge of cross-species variability.
| AAV Variant | Backbone | Engineering Sites | Performance in Mice | NHP-Marmoset | NHP-Rhesus Macaque | Liver De-Targeting |
|---|---|---|---|---|---|---|
| AAV-PHP.B | AAV9 | VR-VIII | Yes | No | No | No |
| AAV-PHP.eB | PHP.B | VR-VIII with flanking substitutions | Yes | 4-fold | No | Yes |
| AAV.CAP-B10 | PHP.eB | VR-IV | Yes | 4-fold | No | Yes |
| AAV.CAP-B22 | PHP.eB | VR-IV | Yes | 12-fold | No | No |
Note: All results are compared with AAV9.
NHPs in AAV Research
Non-human primates (NHPs) are valuable models for AAV capsid evolution and evaluation due to their close similarities to humans in terms of tissue structure and cognitive function. However, one AAV variant selected in one species may not necessarily perform well in another. For instance, AAV.CAP-B22 showed efficacy in marmosets but not in rhesus or cynomolgus macaques (AAVnerGene’s Data). This highlights the importance of using multiple models to validate AAV performance before considering clinical applications.
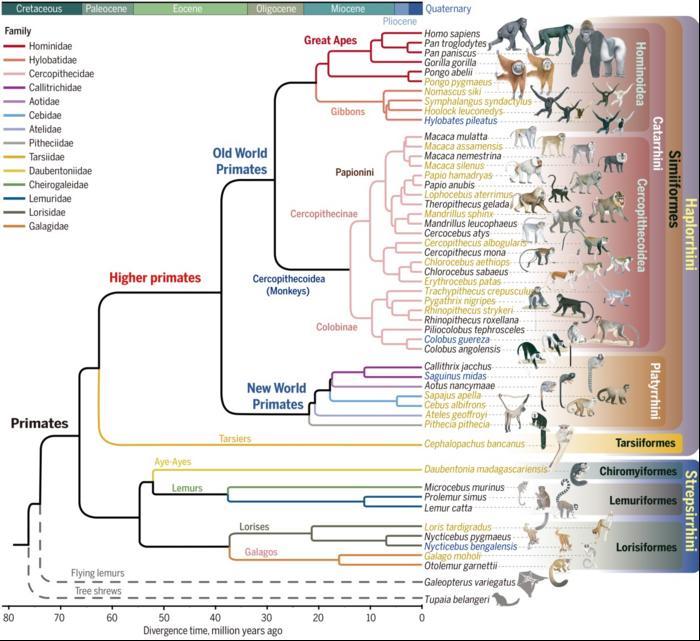
Performance of AAV Variants Across Models
Non-human primates (NHPs) have been proposed as good models for AAV capsid evolution and evaluation due to close similarities to humans in terms of tissue structure and cognitive function. However, one AAV selected from one species may not work in another species, such as AAV.Cap.B22, which is only work in marmoset, but not in rhesus macaques and cynomolgus macaques (AAVnerGene’s Data)
Among the tested AAV variants, AAV9P31 showed the highest activity for crossing the BBB in B6C3 mice at both DNA and RNA levels, with significantly reduced liver expression. Other variants like AAV-F, AAV-MDV1A, AAV-PHP.C2, and PHP.eB also exhibited increased brain transduction and liver detargeting properties. However, the liver-detargeted variants AAV.CAP-B10 and AAV.CAP-B22 did not show improved brain transduction in the B6C3 mouse model. Furthermore, AAV variants selected from NHPs, such as MaCPNS1, MaCPNS2, AAV.Cap-Mac, AAV.cc47, and AAV9P81, also did not perform well in this mouse strain.
AAV Services
AAV Vector Design & Cloning
Expert consultation, design, gene synthesis, and cloning of custom AAV vectors tailored to your research needs.
Learn moreAAV Packaging Service
Reliable, affordable, and fully customizable AAV packaging service with diverse serotype options and high titer.
Learn moreAAV QC & Characterization
Comprehensive quality control assays including qPCR, SDS-PAGE, mass photometry, and next-generation sequencing.
Learn moreOther Viruses at Biohippo
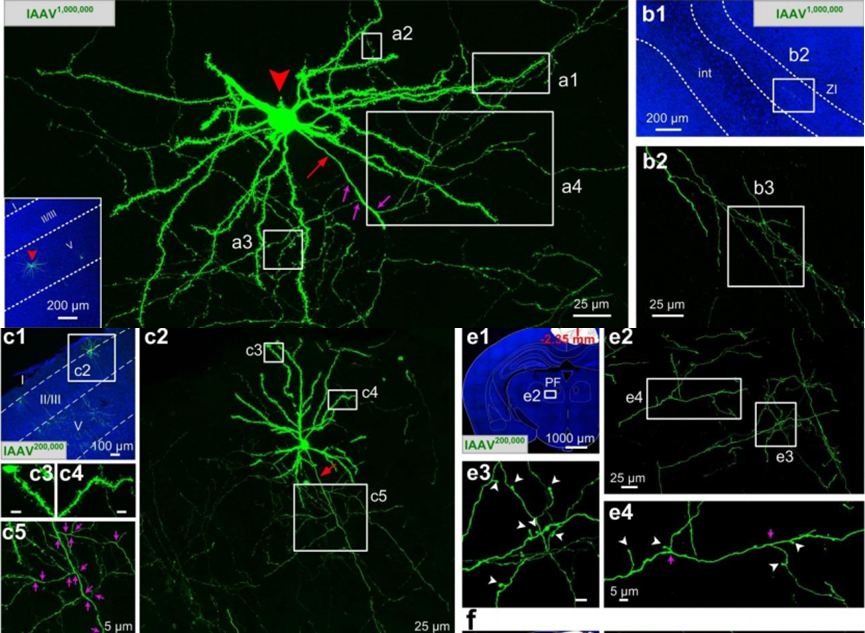
Neural Circuit Tracing
Biohippo offers a variety of viral vectors, including RV, HSV, PRV, and VSV, to replace conventional tracers with the highly efficient, more specific, and less invasive viral method.
Learn More
Lentivirus
Biohippo offers TF reporter lentivirus, immunotherapy lentivirus, validated shRNA, cDNA, and miRNA lentiviruses to meet diverse research needs. We also offer customized service at affodable price.
Learn More Loading ....
Loading ....
