Integrated AAV Offerings: From Cutting-Edge Products to Advanced Custom Services
Overcoming AAV Technology Challenges with Expertly Tailored Vector Design and Production
ATHENA AAV Capsid Platform
In partnership with AAVnerGene, Biohippo presents the ATHENA AAV Capsid Platform, a comprehensive suite of technologies designed to rapidly evaluate, evolve, and develop novel AAV serotypes or variants for specific therapeutic applications. Over the past decade, AAV vectors have emerged as leading gene delivery vehicles for potential gene therapies targeting a range of human diseases. Despite this progress, challenges such as the need for high vector doses and anti-AAV immune responses that result in the loss of vector-transduced hepatocytes have highlighted the limitations of natural AAV serotypes.
To overcome these hurdles, AAV capsid engineering has become a promising strategy to improve the efficacy and safety of AAV vector systems in clinical applications. Engineering new AAV capsids can reduce the required vector dose, lower the risk of immune responses, decrease manufacturing costs, and provide solutions for patients with preexisting immunity to natural AAV serotypes.
The ATHENA platform addresses these challenges by offering two distinct sub-platforms: ATHENA-I, and ATHENA-II, each designed to tackle different aspects of AAV capsid discovery and optimization.
Learn About ATHENA platforms

ATHENA-I Platform: AAV Capsid Evaluation
The ATHENA-I platform is designed for the systematic evaluation of different AAV serotypes or variants using Barcode-Seq technology.
AAV Capsid Barcode Kits: Created using the ATHENA-I platform, these kits allow researchers to assess the efficiency of various AAV capsid variants. Each capsid variant is assigned three different DNA barcodes, and through next-generation sequencing (NGS) of the DNA or RNA levels, researchers can determine the most efficient AAV capsid for their specific applications.
Available Kits: AAVnerGene provides both common and tissue-specific AAV Capsid Barcode Kits based on the ATHENA-I platform. The common kits include 15 widely used AAV serotypes, while tissue-specific kits contain an additional 9 selected tissue-targeting capsids. Custom library services are also available, allowing users to integrate their own reporter systems and specific capsids.

ATHENA-II Platform: AAV Capsid Evolution
The ATHENA-II platform focuses on evolving novel AAV capsids with tissue-specific tropism from high-complexity random peptide insertion libraries.
AAV Capsid Libraries: These libraries are built on the ATHENA-II platform and involve CAP genes with random peptides driven by a CAG/P40 hybrid promoter. The P40 promoter drives CAP expression during AAV production, while the CAG promoter drives CAP expression in target cells or tissues. The random peptides serve as barcodes for enrichment analysis.
Available Libraries: AAVnerGene offers AAV Capsid Libraries with different serotypes, variable regions for peptide insertion, and random peptide lengths. Each library has a complexity of over one billion. Custom library construction, production, and evolution services are also provided.
AAV Services
AAV Vector Design & Cloning
Expert consultation, design, gene synthesis, and cloning of custom AAV vectors tailored to your research needs.
Learn moreAAV Packaging Service
Reliable, affordable, and fully customizable AAV packaging service with diverse serotype options and high titer.
Learn moreAAV QC & Characterization
Comprehensive quality control assays including qPCR, SDS-PAGE, mass photometry, and next-generation sequencing.
Learn moreOther Viruses at Biohippo
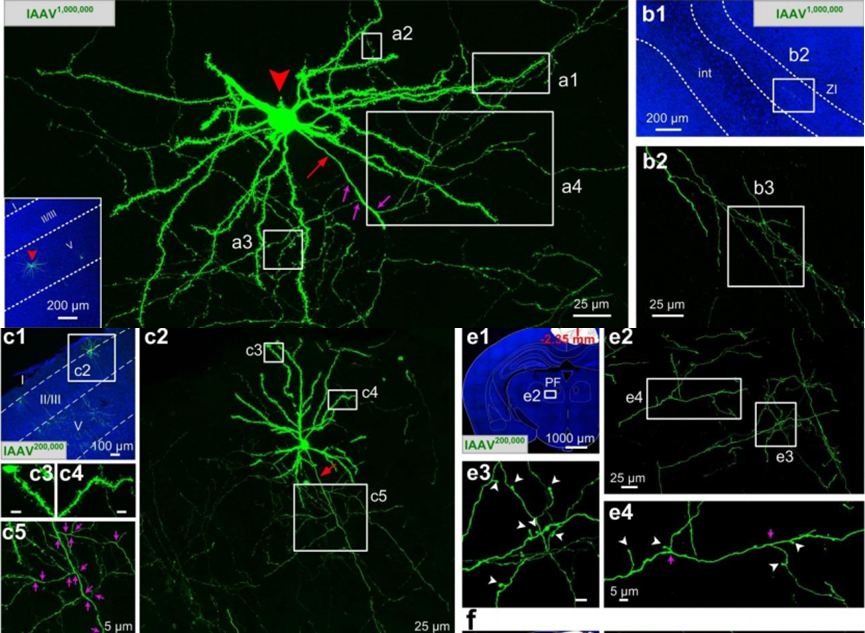
Neural Circuit Tracing
Biohippo offers a variety of viral vectors, including RV, HSV, PRV, and VSV, to replace conventional tracers with the highly efficient, more specific, and less invasive viral method.
Learn More
Lentivirus
Biohippo offers TF reporter lentivirus, immunotherapy lentivirus, validated shRNA, cDNA, and miRNA lentiviruses to meet diverse research needs. We also offer customized service at affodable price.
Learn More Loading ....
Loading ....
